- Product Details
Keywords
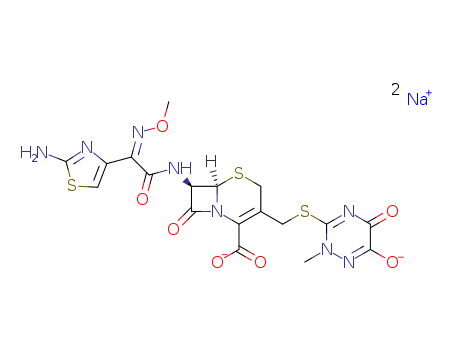
- Ceftriaxone Sodium
- Ceftriaxone Sodium
- Ceftriaxone Sodium
Quick Details
- ProName: Ceftriaxone Sodium
- CasNo: 74578-69-1
- Molecular Formula: C18H16N8Na2O7S3
- Port: Beijing /Shanghai /Tianjin
- ProductionCapacity: Metric Ton/Day
- Purity: 99%
- LimitNum: 25 Kilogram
Superiority
Packaging & Delivery
| Packaging Detail: | 5KG/TIN |
| Delivery Detail: | WITHIN 1 WEEK |
Specifications
Name:Ceftriaxone sodium
CAS:74578-69-1
GMP Manufacturer
Assay: 99%
Sterile powder
Name:Ceftriaxone sodium
CAS:74578-69-1
GMP Manufacturer
Assay: 99%
Sterile powder
high quality,best service,competitive price
Ceftriaxone Sodium Sterile
|
ITEMS |
SPECIFICATION |
RESULTS |
|
Appearance |
White to pale yellow Crystalline Powder |
Passed |
|
Identification |
IR Spectrum HPLC RT Sodium |
Passed Passed Passed |
|
Clarity |
≤1#Clarty standard solution |
0.5# |
|
Crystallinity |
Meets the requirements |
Passed |
|
PH |
6.0~8.0 |
6.5 |
|
Water |
8.0%~11.0% |
9.6% |
|
Sterility |
Sterile |
Passed |
|
Bacterial Endotoxins |
NMT 0.20 USP EU/mg |
Passed |
|
Acetone |
≤0.5% |
0.05% |
|
Viable matter |
≤5 |
Passed |
|
Particulate matter |
≥10um NMT 6000 ≥25 um NMT 600 |
Passed Passed |
|
Assay (HPLC) |
≥795ug/mg (as Ceftriaxone, anhydrous) |
935ug/mg |
|
Storage Condition |
Preserve in tight container. |
|
Details
Ceftriaxone Sodium Sterile
|
ITEMS |
SPECIFICATION |
RESULTS |
|
Appearance |
White to pale yellow Crystalline Powder |
Passed |
|
Identification |
IR Spectrum HPLC RT Sodium |
Passed Passed Passed |
|
Clarity |
≤1#Clarty standard solution |
0.5# |
|
Crystallinity |
Meets the requirements |
Passed |
|
PH |
6.0~8.0 |
6.5 |
|
Water |
8.0%~11.0% |
9.6% |
|
Sterility |
Sterile |
Passed |
|
Bacterial Endotoxins |
NMT 0.20 USP EU/mg |
Passed |
|
Acetone |
≤0.5% |
0.05% |
|
Viable matter |
≤5 |
Passed |
|
Particulate matter |
≥10um NMT 6000 ≥25 um NMT 600 |
Passed Passed |
|
Assay (HPLC) |
≥795ug/mg (as Ceftriaxone, anhydrous) |
935ug/mg |
|
Storage Condition |
Preserve in tight container. |
|